Healing begins with a Single Cell.™
Stemagen News Spotlight
- OCT 5 '11: What the Recent Human Stem Cell "Breakthrough" Really is - and isn't.
- JAN 17 '08: Stemagen First to Create Cloned Human Embryos from Adult Cells
- MAR 5 '07: Stemagen and UPenn Partner to Advance the Therapeutic Use of New and Unique Type of Human Embryonic Stem Cell
- JAN 11 '07: House Passes Bill to Expand Stem Cell Funding
- NOV 17 '06: Australian Senate Approves Embryonic Stem Cell Research
- SEP 12 '06: Stemagen CEO Writes Letter to Governor Regarding Senate Bill 1260
- JUL 17 '06: Stemagen Announces Important Institutional Review Board Approval
- Scientific Publications/Presentations
We have been interested today to read media reports suggesting the research described in the article "Human Oocytes Reprogram Somatic Cells to a Pluripotent State," authored by Noggle et al and published in the journal Nature today, represents an advance over our previously published findings.
In 2008, Stemagen reported the first successful attempt at creating cloned blastocysts from adult human somatic cells using donor oocytes (eggs) from which their genetic material had been removed. Obtaining such blastocysts is the critical step immediately prior to beginning the process of generating clinically useful cloned stem cell lines. The cells from these blastocysts were confirmed to have a normal complement of 46 chromosomes and were confirmed by an independent lab to be true clones, i.e., all nuclear DNA present in the blastocyst generated originated from the somatic cell donor and not the oocyte donor. This research demonstrated the capability of proteins and other factors remaining in the egg after the nucleus is removed are capable of reprogramming somatic cells back to their "embryonic state."
Thus, we were very pleased to read the Noggle et al article today in Nature that described research funded by the New York Stem Cell Foundation (NYSCF). Their results provide strong support for Stemagen's long-held contention that the oocyte is the most effective tool for reprogramming fully differentiated adult somatic cells so they can be used for patient specific stem cell therapies.
In this study, researchers were able to generate two stem cell lines after fusing skin cells (fibroblasts) with intact oocytes (eggs)-ones that that had not had their genetic material removed. Then in a subsequent series of follow-up studies, they were able to show that factors within the egg had apparently completely erased the epigenetic fingerprint that makes a cell a fibroblast. This type of study describes a "cell fusion" procedure in which two nucleated cells are joined together to make a hybrid cell. Because such a hybrid cell differs genetically from its somatic cell donor, the resultant cells cannot properly be considered "clones."
Research from as long ago as 2006 has shown that other pluripotent cells (e.g. embryonic stem cells) are also able to reprogram adult somatic cells when they are fused together. Thus it would have been surprising indeed for this cell fusion experiment, which used an egg, the most effective reprogramming cell in the body, to have been unsuccessful.
The procedure that Noggle et al used to create blastocysts and stem cell lines bears no relationship to the type of research we published in 2008, in which the nucleus of the egg is replaced by a nucleus from a somatic cell.
Thus we have been surprised today to read media reports suggesting this research represents an advance over our previously published findings.
In their article, the researchers describe attempts to accomplish what we have previously reported in 2008, i.e., create normal cloned blastocysts by replacing the oocyte nucleus with that of a somatic cell but, unfortunately, those attempts were unsuccessful with all of their embryos arresting prior to progressing beyond the 12-cell stage (blastocysts have approximately 64 cells).
Samuel Wood, M.D., Ph.D., CEO of Stemagen, comments on the Noggle et al study:
"Since the oocyte and somatic cell were fused without removing the oocyte's genetic material, the resulting blastocysts and stem cell lines were genetically abnormal having approximately 69 chromosomes rather than 46 chromosomes, the normal number for human cells. Because these stem cell lines are genetically abnormal, they are of little or no therapeutic value.
Using the particular protocol they employed, these researchers were, unfortunately, not able to generate the cloned blastocysts required to create a chromosomally normal human embryonic stem cell line. In particular, they were unable to generate blastocysts and early stem cell lines from enucleated oocytes as we have previously reported. In fact, none of their cloned embryos progressed beyond the early embryo stage using this technique. Their lack of success may also be attributed in part to the type of egg donor that they used. Our age cutoff for egg donors was considerably lower and all of our donors had previously been successful reproductive egg donors thus unequivocally demonstrating their ability to create high quality eggs. This is something that cannot be determined simply by looking at the appearance or rate of development of an embryo; Noggle et al assessed their egg quality using this as the criteria.
Utilizing our optimized protocol, we have reported that almost 25% of our cloned embryos became blastocysts. Generating cloned blastocysts using enucleated oocytes is critical so that any stem cell lines that are derived are genetically normal and do not have the cell-surface markers that could cause them to be rejected if used for transplantation.
Despite their lack of success with this critical procedure, their ready source of oocytes and funding from NYStem (New York State Stem Cell Board) makes us hopeful that with time they will be able to modify and optimize their procedures allowing them to create cloned blastocysts that are chromosomally normal and therapeutically useful for transplantation. It is only then that the true therapeutic potential of 'therapeutic cloning' can be determined.
That the research team was able to acquire the eggs necessary for this research is a testament to the New York State Stem Cell Science Board (NYStem), which drafted guidelines allowing women who choose to donate eggs for research to be fairly compensated for their time and discomfort. That there is no difficulty in locating women willing to donate for research is confirmed in this paper since all 16 women who were offered the possibility to donate for stem cell research chose to do so. We had previously reported that over 80% of women who had decided to donate their eggs for reproductive purposes would also do so for stem cell research.
As we go forward as a company, Stemagen will fight vigorously to reverse the illogical and ethically indefensible California guidelines that ask egg donors to accept insufficient compensation for their donations while those who undergo identical medical procedures, experience an identical level of inconvenience, suffer identical levels of discomfort and subject themselves to an identical level of risk for the egg donation process for the purposes of third-party reproduction are compensated in what has been determined to be a fair and equitable fashion by independent national entities like the American Society for Reproductive Medicine. It is unacceptable that women who generously choose to donate eggs for stem cell research with the hope their donations will help millions of people with incurable degenerative diseases should be compensated in such an embarrassingly inadequate fashion. This failure to equitably compensate these women is ethically indefensible and unconscionable.
It is undeniable that Stemagen's ability to move this critically important work forward has been greatly limited because as a California company we are prohibited from providing appropriate and fair compensation for egg donors under SB 1260 and CIRM guidelines. We are also unable to obtain funding from the California Institute of Regenerative Medicine (CIRM), the agency that distributes Prop 71 funds, because as a practical matter, CIRM has chosen not to fund such research until it is clear that an adequate number of high quality eggs will be available to conduct the research.
We therefore call on Governor Brown, the state legislature and CIRM to immediately take the necessary legislative and regulatory steps to provide for the fair compensation of research egg donors, so that arguably the most promising way of creating therapeutically useful stem cells can move forward in California. Failure to do so may unnecessarily prolong the suffering of millions of Americans living with debilitating degenerative diseases for which there are no cures or treatments. Failure to do so is contrary to the will of the more than 7 million voters who made it clear that they wanted therapeutic cloning research to be funded when they passed Proposition 71."
Download complete article here
DOWNLOAD HIGH RESOLUTION IMAGES HERE
Major Advancement Towards Creating Patient-Specific and Disease-Specific Stem Cells For Therapeutic Use
January 17, 2008
LA JOLLA, CA – Stemagen, a privately held embryonic stem cell research company, announced today it has become the first in the world to create, and meticulously document, a cloned human embryo using somatic cell nuclear transfer (SCNT).
Stemagen CEO Samuel H. Wood, M.D., Ph.D., a co-author of the publication and a donor of the cells from which the embryos were cloned, terms this achievement “a critical milestone in the development of patient-specific embryonic stem cells for human therapeutic use, potentially including developing treatments for Parkinson’s, Alzheimer’s and other degenerative diseases.” Stemagen’s research is exhaustively detailed in a paper published in today’s issue of the highly regarded peer-reviewed scientific journal Stem Cells.
“This is not merely a technical improvement on previous research in this area,” said Andrew French, Ph.D., lead author on the paper, “Development of Human Cloned Blastocysts Following Somatic Cell Nuclear Transfer (SCNT) with Adult Fibroblasts.”
“No other scientific group has documented the cloning of an adult human cell, much less been able to grow it to the blastocyst stage, the stage at which it is the adult donor cell that is driving embryonic development, the stage that yields the cells (the inner cell mass) from which embryonic stem cell lines are made,” said French, who is Stemagen’s Chief Scientific Officer.
Five blastocysts were developed from 25 donated mature oocytes. Three were confirmed to be clones based on DNA fingerprinting demonstrating the presence of the skin cell donor DNA in the blastocyst, while one was further confirmed to be a clone by an additional mitochondrial DNA (mtDNA) analysis which revealed the presence of oocyte donor mtDNA without any oocyte donor nuclear DNA. For technical reasons, the genetic material in the remaining two blastocysts did not amplify to the extent required for analysis, and so while it is likely they were clones, the evidence required to claim that with certainty was not present. Thus, in this study, cloned blastocysts were successfully created from approximately 10% of all mature donated oocytes, an unexpectedly high rate given past research in this field.
The oocytes used in this study were donated, without compensation, by egg donors and intended parents undergoing egg donation cycles for reproductive purposes at the Reproductive Sciences Center in La Jolla, a leading fertility center specializing in egg donation and other advanced assisted reproductive technologies.
“As important as stem cell research is, all of us involved in this study realized that our overriding responsibility was to the intended parents who entrusted us with their dream of having a child,” said Catharine Adams, Ph.D., a co-author on the paper and the laboratory director for Reproductive Sciences Center. “We in the IVF laboratory felt comfortable in this collaboration because we have consistently achieved pregnancy rates of greater than 80% from these types of high quality egg donors. In this study, all the intended parents were successful in achieving a pregnancy.”
Stemagen and the Reproductive Sciences Center worked closely, over an extended period of time, with a leading independent Institutional Review Board (IRB) to develop procedures ensuring that all parties received comprehensive informed consent and that procedures were in place to protect their confidentiality in the process. All research procedures, including the culturing of the skin cells (fibroblasts) were performed under clinical laboratory conditions in close cooperation with the Assisted Reproductive Technologies (ART) Laboratory of the Reproductive Sciences Center, directed by Catharine Adams, Ph.D. French notes, “An important reason for the success of our SCNT procedures depended on the close coordination between our laboratory personnel and fertility center laboratory staff. Timing is a critical element in maximizing the probability of success in this type of procedure.”
Wood points out that this research was exhaustively scrutinized by some of the world’s most respected scientists and underwent an exceptionally rigorous process of verification, “This achievement was so critical to our field, we felt we should spare no effort in the process of establishing the validity of our work.”
DNA fingerprinting is the scientifically accepted method for determining if an embryo is a true clone. According to French, “All samples were subjected to this type of analysis to determine their true genetic makeup.”
For that, the company turned to Genesis Genetics, a recognized worldwide leader in the field of reproductive embryonic analysis.
Company founder and CEO, Mark Hughes, M.D., Ph.D., said “We were proud to collaborate with Stemagen in this important accomplishment. As the leading provider of genetic diagnosis of human embryos, it was important for an independent company like Genesis Genetics to be involved in the verification of this achievement.
Stemagen, Inc., is dedicated to the production of patient-specific embryonic stem cells for therapeutic use through SCNT and “uniparental” embryonic stem cells technology.
Download complete article here
Novel embryonic stem cell lines created from unfertilized embryos may be an acceptable alternative for those opposed to embryonic stem cell research
March 5, 2007
LA JOLLA, CA – Stemagen announced today it has acquired the exclusive commercial option rights to a patent for a groundbreaking technique that allows the development of embryonic stem cells appearing to have a markedly enhanced potential for therapeutic use – uniparental embryonic stem cells. Because these extraordinary stem cells are created without fertilization they may represent an acceptable alternative for those who oppose the traditional method that requires the use of embryos that are potentially capable of reproduction.
“Because Stemagen has been successful in developing human uniparental embryonic stem cell lines, we believe we are uniquely positioned to capitalize on this patent,” said Stemagen CEO Samuel H. Wood, M.D., Ph.D.
This announcement follows the publication of a paper in this week’s Genes and Development in which a major breakthrough in the field of cell transplantation is reported by Dr. John McLaughlin and his team of researchers at the University of Pennsylvania. The study describes the successful transplantation of differentiated uniparental mouse cells to rescue mice subjected to lethal doses of radiation.
In conjunction with the agreement for this exclusive patent option, Stemagen is sponsoring a joint research program with Dr. McLaughlin to assess the viability of human uniparental cells in regenerative medicine.
“As a result of this strategic partnership with the University of Pennsylvania, Stemagen now holds exclusive rights to an existing patent that may lead to the therapeutic use of this technology,” said Wood. Traditional embryonic stem cell lines are derived from embryos that result when an egg is fertilized by a sperm.
Because uniparental embryos are derived from the genetic material of either an egg or sperm only (one parent source), they are incapable of being used for reproduction. However, scientists are still able to generate stem cell lines from these uniparental embryos.
“We are excited about this partnership, and believe this intellectual property complements our existing expertise in developing lines of embryonic stem cells for a new era in regenerative medicine,” said Andrew French, Ph.D., Stemagen’s Chief Scientific Officer.
Download complete article here
La Jolla-based Stemagen applauds broad bipartisan support, but measure falls short of veto-proof margin.
January 11, 2007
LA JOLLA, CA – Stemagen today commends the U.S. House of Representatives for overwhelmingly passing House Resolution 3, which would expand federal funding for embryonic stem cell research. While the measure failed to pass with enough votes to override an expected Presidential veto, it did enjoy broad bipartisan support.
H.R. 3 passed 253-174, which, if every member of the House votes, is still 37 votes shy of the two-thirds majority needed to override a promised Presidential veto.
Stemagen CEO Samuel H. Wood, M.D., Ph.D., applauded the bill’s passage. “Even though this bill did not have enough votes in the House to override a veto, we are encouraged that yet another vote has illustrated that this is not a Republican or Democrat issue; it’s a human issue. When this bill passes the Senate and is put on the President’s desk, it is our fervent hope he use a pen to sign it, and not a stamp to veto it.”
On July 19, 2006, after both Houses of Congress passed similar legislation, President Bush used the first veto of his presidency to reject the bill. When the House of Representatives attempted to override the veto, it fell 51 votes shy of the two-thirds majority needed. The issue then too enjoyed broad bipartisan support, with 50 House Republicans voting both in favor of the measure, and in favor of overriding the veto.
“We hope the President will, after considering the widespread public and congressional support for embryonic stem cell research, as well as recently published promising stem cell research, sign this bill following its near certain passage by the Senate, thus allowing the full potential of this extraordinary technology to be realized,” Wood said.
Stemagen is a privately funded biotechnology company in La Jolla committed to harnessing the potential of embryonic stem cells and hastening the day when they may lead to effective therapeutic treatments.
Download complete article here
Stemagen CSO Andrew French, Ph.D., is interviewed by The Age, Melbourne’s Paper of Record on his thoughts regarding a Conscience Vote in the Australian Senate that would, if ratified, allow for embryonic stem cell research in that country.
November 17, 2006
LA JOLLA, CA - For the complete article, please click this link: http://www.theage.com.au/articles/2006/11/10/1162661899454.html
Download complete article here
Stemagen Urges Governor Schwarzenegger to Veto SB1260
September 12, 2006
LA JOLLA, CA – Stemagen, a leading California-based embryonic stem cell research company, is urging Governor Schwarzenegger to veto SB 1260, a pending bill that denies women, but not men, fair compensation for participating in embryonic stem cell research.
“Not only is this bill patently discriminatory against women, its effect will be to dramatically slow progress in one of the most promising areas of embryonic stem cell research–nuclear transfer (‘therapeutic cloning’),” said Samuel H. Wood, M.D., Ph.D., and CEO of Stemagen.
This bill devalues the contribution of women who choose to participate in embryonic stem cell research through egg donation by denying them the reasonable compensation customary for subjects participating in biomedical research. “Men, however, are not prohibited from receiving compensation for participating in this same type of research,” Wood asserted.
Stemagen believes this denial of fair compensation for these women will dramatically slow progress in the field of embryonic stem cell research since few women will choose to participate if they will not be appropriately compensated for doing so. “The signing of this bill will help no one and will delay the day Californians can benefit from the therapeutic potential of the most promising science of the new millennium–the research they overwhelmingly voted to fund under Proposition 71.
“Before the Governor today is a bill that will codify the inequitable treatment of women who choose to advance embryonic stem cell research,” Wood said.
“Obviously, both sperm and eggs are required to create embryos,” Wood notes. “If this bill is signed, men can be compensated for donating sperm for stem cell research. By what twisted logic would women be denied compensation for donating eggs?”
Wood illustrates his position by pointing out that men are routinely paid for sperm donation for research purposes. Wood says, “The FDA specifically approves of payments to research subjects, terming them ‘a recruitment incentive’”:
“Subjects are sometimes paid for their participation in research, especially in the early phases of investigational drug, biologic or device development. Payment to research subjects for participation in studies is considered a recruitment incentive. Financial incentives are most often used when health benefits to subjects are remote or nonexistent. Volunteers may be offered compensation in certain trials for their time, and for discomfort that may be experienced during the trial. The amount of compensation is determined by the amount of time you will be required to dedicate to the trial, and to the level of discomfort that might be associated with medical or surgical procedures related directly to the study.”
The American Society of Reproductive Medicine (ASRM) has published guidelines (Fertility and Sterility, July 2005) for appropriate compensation women donating eggs:
“… oocyte donors spend 56 hours undergoing interviews, counseling and medical procedures related to the process [while] men take one hour.”
Thus they point out, it is reasonable and fair for women to be compensated far more than men for their donation. This time analysis does not take into account the experience of donating eggs is far different to that of donating sperm. Wood explains women undergo a two-to-three-week process of daily shots, dietary modifications, culminating in a surgical procedure requiring anesthesia to harvest the eggs, followed by a two to four week period of time in which the ovaries gradually return to normal.
The highly negative consequences of passing a bill such as this can clearly be seen based on recent events in Massachusetts. For several years, Massachusetts had been a leader in the most advanced area of embryonic stem cell research, nuclear transfer (featured in a cover story for Scientific American in January 2002). That all changed in May 2005 when the Massachusetts Legislature overrode a veto by Governor Romney of a bill similar to SB 1260 (Massachusetts’ Senate No. 2039) and it became law. Since then, even prestigious research institutions such as Harvard’s School of Medicine have had no success in recruiting egg donors and, not surprisingly, the company that performed some of the most important early research in this area has moved its headquarters from Massachusetts to California.
“If California makes the same mistake as Massachusetts, our ability to advance this research will no doubt suffer the same tragic consequences,” Wood says. “This bill fails to recognize the commitment, to say nothing of the significant discomfort, women endure to donate their eggs,” Wood said. “If SB 1260 is signed into law, it will effectively say that we as a society value the minimal effort of a sperm donor more than the far more significant contribution of an egg donor.”
Download complete article here
San Diego-based Stemagen receives approval for groundbreaking stem cell research. Privately-funded company receives independent IRB approval for therapeutic cloning.
July 17, 2006
LA JOLLA, CA – Stemagen, a company dedicated to the production of therapeutic stem cells, announced today it has received Institutional Review Board (IRB) approval to develop embryonic stem cells using a comprehensive set of methods, including the process of Somatic Cell Nuclear Transfer (SCNT) for “therapeutic cloning.” Stemagen also received approval to use excess fresh eggs, which have shown in previous research to offer the greatest chance of successfully completing the therapeutic cloning process.
“This landmark IRB approval paves the way for Stemagen to test a new method of producing human stem cells,” said company CEO Samuel Wood, M.D., Ph.D. “We believe this will lead to disease-specific stem cell lines designed to speed the delivery of effective treatments for millions suffering from incurable diseases.”
With this announcement, Stemagen becomes the first in San Diego and one of only a handful of entities in the world to have received independent IRB approval for this promising, but challenging, technology.
“Embryonic stem cells are unique because they are able to self-renew and be directed to become any one of the body’s 220 different cell types,” said Andrew French, Ph.D., Stemagen’s Scientific Director and one of the world’s leading experts on cloning procedures, “We believe they can be a vital component in helping develop treatments for diseases such as Alzheimer’s, Parkinson’s, traumatic spinal cord injury, diabetes, heart disease, rheumatoid arthritis, and hearing and vision loss.”
Stemagen has also received IRB approval to develop human embryonic stem cells from excess embryos donated by couples who would otherwise have chosen to discard them. Wood, who is also one of the country’s leading fertility specialists, said, “After a couple has completed their family using procedures like in vitro fertilization (IVF), it is common to have excess frozen embryos in storage, and unfortunately the vast majority of couples choose to discard them because they are uncomfortable with donating them to other couples for reproductive purposes. The ability to donate these embryos to an ongoing stem cell research program gives these couples a viable alternative to simply discarding them.” There are currently an estimated 400,000 frozen embryos in storage at fertility clinics, hospitals and other locations in the United States.
As is required by California law, no egg or embryo donor will be compensated (beyond expenses and lost wages) for donations to stem cell research. “Although finding willing egg donors under these financial constraints is challenging, the response from couples with unneeded embryos has been gratifyingly positive,” says Wood.
Because Proposition 71 funds are still tied up in litigation and because federal funding is not available for this type of research, Stemagen is privately funded. “We realized we could not, in good conscience, wait for Proposition 71 to wind its way through the California legal system, says Wood. “Along with the research being done at UCSF, we believe this is the beginning of the process of fully realizing the promise of Prop 71.”
“Making sure this research is performed in a highly ethical and transparent manner was our highest priority,” says Wood. To work through the many ethical issues involved in this type of research, Stemagen chose to work with one of the most respected independent human subjects protection programs in the country, Independent Review Consulting, Inc (IRC), headquartered in Northern California. “We are grateful for the several months of effort expended by IRC as we fine-tuned all aspects of our research protocols to ensure that subjects’ rights would be fully protected,” says Wood.
September 18, 2007
from Stem Cell Reviews, Volume 2, 2006Human Therapeutic Cloning (NTSC) Applying Research From Mammalian Reproductive Cloning
Andrew J. French,*,1 Samuel H.Wood,1,2 and Alan O.Trounson3
1Stemagen Corporation, La Jolla, California, USA 92037; 2The Reproductive Sciences Center, La Jolla California, USA 92037; and 3Monash Immunology and Stem Cell Laboratories, Monash University,Victoria 3800 Australia
*Correspondence and reprint requests to: Andrew French, Stemagen Corporation, La Jolla, CA 92037. E-mail: afrench@stemagen.com
Abstract
Human therapeutic cloning or nuclear transfer stem cells (NTSC) to produce patientspecific stem cells, holds considerable promise in the field of regenerative medicine. The recent withdrawal of the only scientific publications claiming the successful generation of NTSC lines afford an opportunity to review the available research in mammalian reproductive somatic cell nuclear transfer (SCNT) with the goal of progressing human NTSC. The process of SCNT is prone to epigenetic abnormalities that contribute to very low success rates. Although there are high mortality rates in some species of cloned animals, most surviving clones have been shown to have normal phenotypic and physiological characteristics and to produce healthy offspring. This technology has been applied to an increasing number of mammals for utility in research, agriculture, conservation, and biomedicine. In contrast, attempts at SCNT to produce human embryonic stem cells (hESCs) have been disappointing. Only one group has published reliable evidence of success in deriving a cloned human blastocyst, using an undifferentiated hESC donor cell, and it failed to develop into a hESC line. When optimal conditions are present, it appears that in vitro development of cloned and parthenogenetic embryos, both of which may be utilized to produce hESCs, may be similar to in vitro fertilized embryos. The derivation of ESC lines from cloned embryos is substantially more efficient than the production of viable offspring. This review summarizes developments in mammalian reproductive cloning, cell-to-cell fusion alternatives, and strategies for oocyte procurement that may provide important clues facilitating progress in human therapeutic cloning leading to the successful application of cell-based therapies utilizing autologous hESC lines.
Index Entries: Cloning; embryonic stem cells; human; oocyte donation; somatic cell nuclear transfer; therapeutic cloning.
Introduction
Human “therapeutic cloning” describes the use of somatic cell nuclear transfer (SCNT) to produce patient-specific stem cells. If scientific advances allow therapeutic cloning to be performed in a consistent and efficient manner, its potential in understanding and developing treatments for degenerative diseases is potentially limitless. Given the recent voluntary withdrawal, amid allegations of scientific impropriety, of publications that claimed successful human therapeutic cloning (1), it would seem an opportune time to regroup and to review the available data from mammalian reproductive SCNT research that may ultimately suggest new strategies to progress the goal of therapeutic cloning in humans. As a starting point, it is useful to reexamine the parallels and recent developments in mammalian reproductive cloning that may ultimately provide the conduit to the promise of safe and effective patient- specific pluripotent cells for cell therapies, and tissues for regenerative medicine (2–8). Human nuclear transfer (NT) remains in its infancy, with the one reliable report demonstrating the generation of a single cloned blastocyst from an undifferentiated human embryonic stem cell (hESC) donor cell, and it failed to reinitiate into a hESC line (9).
This review highlights recent strategies in mammalian biology that improve the efficiency of nuclear transfer and nuclear reprogramming. These strategies include the use of pluripotent cells as donor cells and the treatment of donor cells with cellular extracts to increase the access of reprogramming factors to donor chromatin. Potential approaches to the challenging problem of procuring high-quality oocytes for use in the generation of cloned human embryos is the critical first step toward the accomplishment of SCNT for patient-specific ES cells.
Reproductive Cloning
The restoration of totipotency to a differentiated nucleus following fusion with an enucleated oocyte, to produce healthy cloned animals, has been progressively applied to an increasing number of animal species using a variety of donor cell types (10–13). Although remarkable, the process is highly inefficient and subject to epigenetic errors that result in high rates of morbidity and mortality throughout development. Although the potential applications of SCNT in research, industry, and animal breeding are vast (10,14–19), they remain hampered by the requirement to achieve competitive commercial outcomes. To date, SCNT, in combination with transgenic strategies, has been applied to laboratory and domestic animals in the areas of:
- Basic research to generate animal models of human disease to further our understanding of biological events surrounding development and functional genomics;
- Animal breeding programs to increase biological efficiency at a reduced cost;
- Preclinical testing of new therapeutic interventions for human medicine; and
- Genetic rescue of endangered mammals by cross-species SCNT.
To achieve full-term development, the cloned embryo has to overcome a significant number of molecular and cellular challenges, including the regulatory environment of the recipient oocyte, the plasticity of reprogramming in the donor cell, recipient and donor cell cycle coordination, technical competence of micromanipulation, chromatin remodeling and reprogramming of gene expression, parthenogenetic activation of the reconstructed cell, and exposure to culture conditions used to reinitiate embryonic development.
The rate of generation of cloned offspring by nuclear transfer remains low in all species, irrespective of donor cell type (20–23). In many species, and within strains of species, it remains technically challenging to produce cloned offspring. Conversely, nuclear transfer in the mouse and bovine has been used successfully to generate ESC lines from somatic cells with relative ease using a variety of genotypes and cell types from both genders (24,25). These ESCs have been used to rescue immune-deficient phenotypes (26); however, it remains to be determined if ESC lines have the same functional characteristics as those derived from fertilized embryos. Recent evidence in the mouse suggests a high degree of equivalency between nuclear transfer stem cell (NTSC)-derived ESCs and those from fertilized mouse blastocysts (27). Collectively, the data indicates that the generation of ESCs from NTSC (therapeutic cloning) is far more efficient (up to 10 times higher in the mouse) and hence less exacting, than the demands for complete development to term of SCNT reconstructed embryos (28–32).
Mammalian SCNT
Although significant variations exist, the basic features of SCNT in both laboratory and domestic animals involve the trans-plantation of a diploid nucleus into a mature oocyte cytoplast. The procedure was first successfully accomplished in mammals in 1996 with the birth of a cloned lamb (33) that established that cell differentiation and commitment to end-stage tissue type can be reversed to enable complete resetting of the embryonic developmental program. To date, reproductive cloning has been successfully used to derive offspring in 12 species using approx12 different donor cell types from among the approx 210 adult differentiated cell types that exist in mammals (22). Some cell types, including fetal fibroblasts (11,34), ESCs (35–37), andoviduct epithelial cells (38), appear more suitable as donor nuclei for generating offspring than others, although this hypothesis has not been conclusively established (39,40).
Around 6% of all embryos transferred to the synchronized reproductive tracts of surrogate mothers result in healthy long-term surviving clones (41). The majority of cloned embryos fail to thrive as a result of developmental anomalies in both the fetus and placenta (see Fig. 1). These developmental abnormalities are likely to be as a result of aberrations in gene regulation mechanisms, including errors of epigenetic reprogramming of the donor genome following nuclear transfer (35,36,42–44) and are later manifested as precocious or absent patterns of gene expression during development. Abnormalities have been detected in DNAmethylation (44–46), chromatin modification (47), X-chromosome inactivation (48,49), and expression of imprinted and nonimprinted genes (50–56). Even apparently healthy cloned animals show epigenetic defects affecting the expression of genes activated later in development or adulthood (57).
A likely source of variability may be species-specific, including but not limited to speed of development, response to parthenogenetic activation stimuli, and differing metabolic requirements during in vitro culture, or they may derive from variations in the methodologies used. The progeny from clones appear to be free of both developmental and phenotypic anomalies (41,54). The physiology of surviving postpubertal cloned bulls and the quality of their collected semen apparently share equivalent reproductive potential to their original cell donor with no evidence of any deleterious effects in their progeny (58–60). Although confirmation is required at the molecular level, it appears epigenetic errors prevalent during in vivo development of cloned animals are subsequently erased during gametogenesis, preventing transmission of aberrant cloning phenotypes to the offspring of clones.
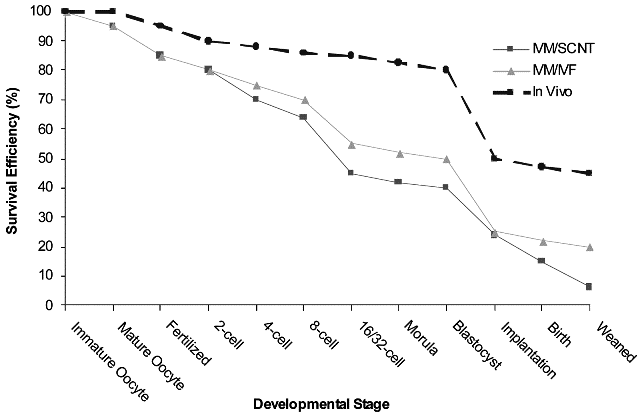
Fig.1. Developmental competence of in vivo, IVM/IVF, and IVM/SCNT bovine embryo production systems.
The reasons for the apparent markedly reduced developmental competence of SCNT embryos when compared with in vivo embryos and those from other assisted reproductive technologies such as in vitro fertilization (IVF), has been the subject of considerable scientific debate. Perhaps unexpectedly then, a direct comparison of the in vitro development rates of cloned vs IVF embryos may serve to clarify this matter (25,61). Although variation between laboratories and subtle differences between species impact the overall success of reproductive cloning, it appears that once optimal conditions have been established in any given laboratory, the in vitro development of both cloned and parthenogenetic embryos approaches that of in vitro fertilized embryos (see Fig. 2; 25,61) and both may be used to derive ESCs. Thus, it may be that attaining the “Gold Standard” for in vitro fertilized embryos will emerge as the key indicator of proficiency in nuclear transfer programs.
SCNT Methodologies
SCNT is a technically demanding, multistep procedure with the potential for cumulative errors that can impact develop-mental potential and each stage of development. Although numerous variations exists, the basic features of the SCNT procedure, used in both laboratory and domestic animals, have remained the same since nuclear transfer in mammals was first performed in the early 1980s and can be summarized as follows:
- Enucleation of the oocyte to form a cytoplast;
- Merger of the donor cell or nucleus with the cytoplast to form a reconstructed one-cell embryo;
- Parthenogenetic activation either before of after reconstruction of the reconstructed one-cell embryo; and
- Preimplantation embryo in vitro culture.
Maternal chromosomes are most commonly removed (enucleated) by aspirating or extruding a membrane-bound portion of cytoplasm containing the metaphase plate, or by bisection containing the metaphase plate. Other methods of enucleation include centrifugation, inactivation, or destruction by ultraviolet or laser irradiation, or noninvasively expelled by chemically induced extrusion (11). Successful enucleation is confirmed by incubating the oocyte in Hoechst33342, a nontoxic DNA interchalating agent, which fluoresces in the presence of ultraviolet light (11). Reconstructed one-cell embryos are formed when the donor cell or karyoplast is deposited into the perivitelline space and adjacent membranes are fused by exposure to a series of electrical pulses, inactivated Sendai virus, or treatment with chemicals such as poly-ethylene glycol. Alternatively, donor nuclei have been directly injected into the cytoplasm of the cytoplast in manner akin to intracytoplasmic sperm injection (ICSI).
Oocyte Source and Availability
The rapid progress seen in optimizing the reproductive cloning of domestic animals was in no small part the result of the availability of an alternative and almost unlimited supply of developmentally competent oocytes; those harvested from ovaries opportunistically scavenged from abattoir processing. There is little doubt that the quality of the oocyte, i.e., its intrinsic developmental potential is a key factor determining the proportion of in vitro fertilized or nuclear transfer embryos that grow to the blastocyst stage (62), and it also influences the overall success of any assisted reproductive technology. An eloquent demonstration of this effect was shown when the super ovulation protocol in the bovine was manipulated to produce an almost maximal number of competent cumulus–oocyte complexes (COCs) as determined by blastocyst formation and significantly improved in vitro embryo production systems (63).
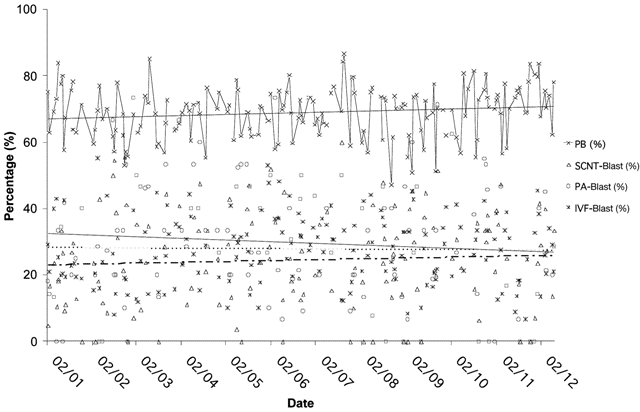
Fig.2. SCNT proficiency in the bovine—a retrospective profile of in vitro production systems to the blastocyst stage.
A yearly profile of in vitro produced bovine blastocysts showing percents of in vitro matured (presence of polar body
[PB], metaphase II), in vitro fertilized (IVF blastocyst rate), parthenogenetically activated (PA blastocyst rate), and
cloned (SCNT blastocyst rate). Linear trend lines shown for each of the four groups (ivm – solid; parthenogenetic
– solid; IVF – dotted; SCNT – dashed lines). Modified from ref.61.
SCNT Improvements
Cell Synchronization
Coordination of the cell cycle between the donor cell nucleus (karyoplast) and the recipient cell cytoplasm (cytoplast) is essential to minimize DNA damage and to maintain euploidy in the reconstructed cloned embryo (64). Cloned offspring have been obtained using cytoplasts at telophase (second meiotic division) and interphase of the first cell cycle and at metaphase II (second meiotic division) (64,65). The readily available metaphase II cytoplasts have typically become the recipient of choice for SCNT experiments.
Cell cycle inhibitors have been used to increase cell cycle synchronization. Recent work in the bovine has shown that donor cells treated with a new metaphase arrestor (2-methoxyestradiol) permitted increased recovery of mitotic cells following shaking. When these cells, arrested at early G1, were subsequently used for nuclear transfer, the resulting SCNT embryos were shown to have a higher developmental competence (blastocysts and live birth rates) (66).
Chromatin Transfer
The general consensus of laboratories performing reproductive cloning in animals is that future improvements are most likely to come from a greater understanding of the molecular mechanisms of reprogramming. In the bovine, a novel system allowing remodeling of mammalian somatic nuclei in vitro before SCNT has been developed (67). Donor cells are permeabilized to allow a mitotic extract access to the chromatin and to facilitate removal of nuclear factors solubilized during chromosome condensation. The condensed chromosomes are transferred into enucleated oocytes before activation. When compared with nuclear transfer embryos, these chromatin transfer embryos appear to exhibit a pattern of markers that more closely resembles those of normal IVF embryos.
Initial results indicate that calves born after chromatin transfer may have a greater survival (see Fig. 3; A French unpublished observations). More importantly, this technique provides a method for directly manipulating the somatic donor chromatin before transplantation, and thus it may be a useful tool to investigate mechanisms of nuclear reprogramming and to enable improvements to the efficiency of both reproductive and therapeutic cloning.
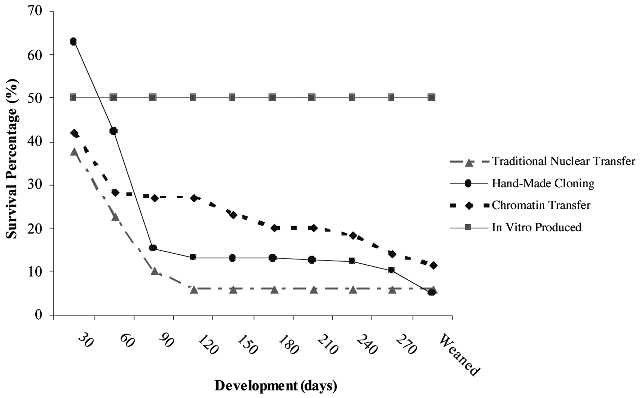
Fig.3. The efficiency of a generation of bovine SCNT calves using traditional nuclear transfer, chromatin transfer,
and hand-made cloning methodologies. The developmental competence of bovine SCNT embryos from a number
of somatic fibroblast cell lines using traditional nuclear transfer, hand-made cloning, and chromatin transfer
methodologies against IVF embryos from the same laboratory (2003–2005; A French unpublished observations).
Therapeutic Cloning
There is a clear distinction between reproductive cloning and the derivation of embryonic cells for their potential use in regenerative medicine. Human reproductive cloning is not only unethical, it is illegal throughout much of the world. Therapeutic cloning, on the other hand, is becoming increasingly accepted with substantial governmental funding available in some localities. These promising initiatives have been guided by principles (68,69) that address the associated wide-ranging ethical and societal concerns (70–73), that may ultimately permit the accrual of benefits to society though improvements in regenerative medicine. Undoubtedly, continued research designed to improve the efficiency of reproductive cloning in laboratory and domestic animals, and to develop alternate somatic cell reprogramming techniques, will prove useful in parallel studies of methodologies to increase the efficiency of patient-specific stem cell strategies.
Therapeutic Cloning in Animal Models
In 2000, the utility of therapeutic cloning was first demonstrated in the mouse, when autologous ESC were derived from a cloned mouse blastocyst (74,75); this observation was subsequently reaffirmed in a number of similar publications (26,28,76). Although autologous cell transplantations using isogenic ESCs have not been reported, synergic transplantations have elicited a partial rescue of a deficient phenotype (26). Homologous recombination in the Rag 2–/–allele was used to correct the genetic defect in a nuclear transfer embryonic stem cell line derived from this immune-deficient mouse. Partial rescue of the phenotype was achieved following transplantation of hematopoietic precursors differentiated from the corrected nuclear transfer ESC line.
Therapeutic Cloning in Human and Nonhuman Primates
At present, only one reliable published report describing the production of a human NTSC blastocyst exists. This blastocyst was derived following fusion of an undifferentiated ESC (9) with an oocyte obtained from a follicular reduction procedure. Unfortunately, this blastocyst failed to develop into an ESC line. Importantly, this report suggested that the oocyte source and age may influence NTSC outcomes. Other preliminary reports have generated only early cleavage stage nuclear transfer embryos (77b) and using cytoplasts obtained from aged human IVF oocytes that had failed to fertilize (77a). Lu et al. (2003) have also reported to have cloned human embryos using fetal fibroblasts and granulosa cells, although the reliability of the finding is uncertain since no DNA fingerprinting or other genetic confirmation demonstrating that they were indeed clones was provided (77c).
The generation of SCNT blastocysts in nonhuman primates appears to be far more inefficient as compared with other species, and attempts at therapeutic cloning have been entirely unsuccessful. The failures to date have been attributed to misaligned chromosomes and defects in microtubule kinetics resulting from removal/interruption of microtubule proteins (nuclear mitotic apparatus protein [NuMA] and kinesin-related protein [HSET]) at the time of enucleation (78,79). However, primate embryonic NT has been used successfully to derive offspring (80). The establishment of therapeutic cloning in nonhuman primates could be important for investigating the effects of undifferentiated and differentiated ESCs in transplantation studies and may allow extrapolation to human therapeutic intervention. Potential strategies for the derivation of human ESC lines for cell-based therapies are shown in Fig. 4.
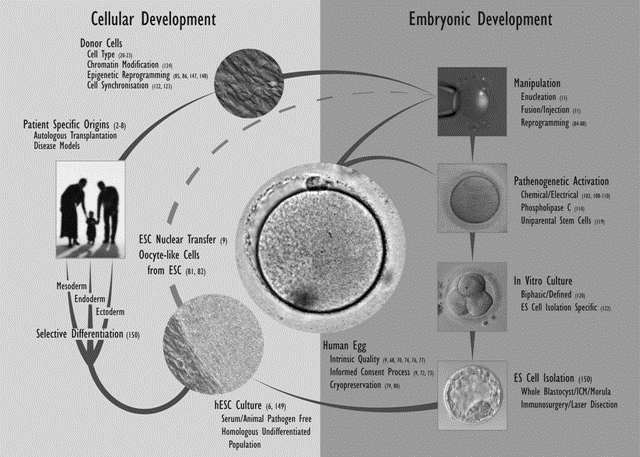
Fig.4. ESCs for cell-based therapies.
Limitations to Human Therapeutic Cloning
Source of Human Oocytes
Undoubtedly, a significant impediment to progress in the area of human therapeutic cloning is the very limited availability of high quality human oocytes for this purpose. In the human, the emergence of IVF as a highly successful treatment option for infertility has given rise to 25 yr of experience in ovarian stimulation (81).
The ability in the human to induce multiple dominant follicles by gonadotrophin stimulation and to grow and produce mature, developmentally competent oocytes has improved the chance of conception both in vivo (empirical ovarian stimulation with or without intrauterine insemination) and with IVF (82). Ovarian stimulation permits the retrieval of numerous COCs and thus compensates for inefficiencies in oocyte maturation, fertilization, in vitro culture, and subsequent in vivo development that might result from having these events occur in vitro rather than in vivo (81).The success of SCNT will very likely be influenced by the age and fertility status of the woman donating her oocytes, with younger women without a history of infertility presumably being the best donor candidates. Although ethical and legal considerations strongly impact the availability of donor oocytes, several prospects exist for consenting patients to provide oocytes for compelling research proposals. To date, sources of donated oocytes for research have ranged from altruistic oocyte donation in which no cost or benefit accrues to the donor (9) to a more traditional oocyte donation model in which donors receive compensation, either monetary or in the form of reduced treatment fees. Although compensation in excess of expenses is controversial and prohibited by law in some localities, a level of compensation that is not viewed as an undue inducement might prove to be practically necessary to obtain the quantity of oocytes necessary for research in this area. As has been prescribed for reproductive oocyte donation, this compensation, if allowed, would acknowledge the very real fact that ovarian stimulation and oocyte retrieval are not without risk or discomfort (83,84).
Source of donated of oocytes for the purposes of SCNT mayinclude the following categories:
- Oocytes that fail to fertilize following either IVF or ICSI. However, no data exists at present to support evidence that aged oocytes, or those that have failed to fertilize in IVF or ICSI, provide a suitable ooplasm for reprogramming donor nuclei (9,77), their utility may be restricted to proficiency testing;
- Oocytes recovered following follicle reduction, a procedure performed to minimize the risk of multi-fetal pregnancy following unintended excessive gonadotrophic stimulation during infertility treatment;
- Immature oocytes obtained from ovarian tissue following oophorectomy, hysterectomy, and caesarian section; and
- Oocytes obtained after ovarian stimulation and oocyte retrieval that are excess to reproductive requirements (9).
There is a need in some of these categories for effective in vitro maturation protocols to be optimized for human oocytes, so that immature oocytes can complete their normal maturation at a practical rate, thus allowing fertilization and early preimplantation embryonic development to occur. Immature oocytes recovered opportunistically at surgery and those recovered as a proportion of COCs following ovarian stimulation for IVF, also require in vitro culture for cytoplasmic maturation to be completed. Immature human oocytes obtained from patients undergoing gynecological surgery or ovulation induction can be matured and fertilized in vitro (82). Following transfer of these embryos to the patient’s uterus, pregnancies and live births have been achieved (85). However, the developmental capacity of in vitro matured oocytes is reduced compared with those retrieved after maturation in vivo (86,87). An alternate source, one that involves the nurturing of immature oocytes from fetal ovarian tissue (88), is unlikely to have ethical support.
As the technology of human SCNT matures and the application of vitrification techniques for oocyte cryopreservation is increasingly adopted (89), it is foreseeable that cryobanks of human ova available for donation could be established to provide a readily available source of cytoplasts for NTSC and therapeutic purposes (90). An alternative supply of human oocytes for NTSC could come from the differentiation of ESCs into germ stem cells and putative ova in vitro. Preliminary studies in the mouse have shown that oocyte-like cells can be produced following differentiation of mouse ESCs (91,92). It does appear that functioning oogonia express markers restricted to this cell type (91,92), although confirmation of their ability to fertilize and produce viable young will be required to fully confirm this potential as a source of reprogramming cytoplasts (93).
Reprogramming and Remodeling
Critical to successful SCNT is the resetting or reprogramming of the epigenetic modifications that maintain the differentiated state of the somatic genome, a state conserved by DNA methylation and by the covalent modification of nucleosome histones. For embryonic development to proceed, the oocyte cytoplasm must interact with the transferred donor nuclei to silence expression of its somatic cell-specific genes and up-regulate embryonic-specific genes in the correct spatio-temporal manner. Although relatively little is known of the cellular factors responsible for remodeling or reprogramming, studies in both Xenopus (94) and mammals (95) have revealed a role for nucleoplasmin in the removal of histones and for the imitation switch in the removal of TATA-binding proteins (TBP) from DNA (96). The mechanisms governing nuclear reprogramming appear to be widely conserved with oocyte components from different species having the ability to reprogram gene expression in a variety of somatic cells from other species (97). The remodeling of nuclear structures by oocyte components results in the reactivation of genes associated with pluripotency in early embryos and pluripotential stem cells (98). The failure or incomplete silencing of gene products that relate specifically to the donor cell types can generate profoundly altered characteristics that lead to severe consequences for the development of cloned embryos (99).
Mitochondrial Heteroplasmy
The unfertilized mammalian oocyte contains approx 100,000 mitochondria. Following fertilization and during early cleavage development, the small number of paternally derived mitochondria, transferred with sperm, are eliminated by an as yet unknown mechanism. The activity of ubiquitin, and the lysosomal apparatus of the egg appear to be involved in the proteolytic destruction of bovine sperm mitochondria inside egg cytoplasm, although the mechanism of ubiquitination of sperm mitochondria leading to their destruction appears species specific (100). By the time the blastocyst stage of development is reached, cells contain approx 100 mitochondria (indicating there is no replication) of entirely maternal contribution (101,102). Rarely, however, mitochondrial heteroplasmia does occur in humans (103) and in other mammals (102,104).
Mitochondrial heteroplasmy has been observed following SCNT in other mammalian species (105–107), although not in all cloned offspring (108,109). The modified somatic cell nuclear transfer technique, termed “hand-made cloning,” involves the construction of several fused cytoplasts so that the SCNT blastocyst, although genomically identical, could have mitochondrial DNA contributions from two to six oocytes (110). In an experiment conducted in the bovine, there was no evidence of any harmful effect of mitochondrial DNA heteroplasmy or differences in reproductive efficiency when compared with the standard nuclear transfer procedure using a single oocyte (110). There is a possibility that species specific or methodological differences could exist that could lead to variations in the level of mitochondrial heteroplasmy that may have implications for human therapeutic cloning and this warrants further investigation (111,112). A recently described ubiquitination method may represent a mechanism for the elimination of paternal mitochondria during fertilization in some species (100).
Parthenogenetic Activation
Critical to the reprogramming of the donor nucleus and the development of NTSC embryos is the resumption of meiosis and restoration of cell cycle progression (113). During normal fertilization, sperm penetration activates the phosphoinositide pathway (114,115), thereby triggering Ca(2+) release and egg activation through a prolonged series of intracellular free calcium ion oscillations that continue for several hours. Although the precise signaling pathway initiated by the sperm remains unknown, recent evidence favors the delivery of a soluble protein factor by the sperm that increases the production of inositol 1,4,5-triphosphate, which acts to open Ca(2+) channels in the endoplasmic reticulum thereby releasing Ca(2+) into the cytosol.
A variety of electrical and chemical activation methods that influence maturation promoting factor activity and mitogen activated protein (MAP) kinase activity have been devised to duplicate or closely mimic the changes in the oocyte cytoplasm that normally are triggered by the sperm during fertilization (116). However, most treatments cause only a single transient Ca(2+) spike, which serves to activate only a fraction of target oocytes, one that increases with the time after ovulation. Attempts to prolong the Ca(2+) oscillations have been successful with less mature oocytes but have been unwieldy (117,118).
A popular and highly effective oocyte activation method involves inducing an elevation in intracellular Ca(2+) with a calcium ionophore while maintaining low levels of maturation promoting factor using a protein synthesis inhibitor, for several hours after the initial Ca(2+) elevation. Regardless of the activation procedure utilized, however, both pregnancy and survival rates after birth of cloned animals remain low. Parthenogenetic activation is therefore likely to be a cocontributor to the inefficiency of cloning. New activation protocols that more closely mimic fertilization and the physiological actions of sperm may yield improvements in viability.
Human oocytes, like those of other mammals, can be artificially activated by stimuli that elevate cytosolic Ca(2+) levels, with both fresh and aged oocytes subsequently undergoing pronuclear formation and early cleavage division (119). However, as other studies have found that calcium ionophore treatment alone does not activate human oocytes in a reliable fashion (120,121), the commonly used activation protocols in human studies also combine a calcium ionophore along with protein synthesis or protein kinase inhibitors (122–124). Although these combination treatments have induced pronuclei formation in activated human oocytes, the success rates in terms of preimplantation development to the blastocyst stage are still very poor when compared with embryo development after in vivo or IVF.
Cytosolic extracts from sperm can cause Ca(2+) oscillations in a range of different mammalian oocytes, including humans (114,125,126). In a further refinement of this approach, Saunders et al. (127) isolated a novel protein from sperm that is a specific isoform of phospholipaseC (PLC), referred to as PLCζ(zeta) (128), which is responsible for generating Ca(2+) release and inducing InsP3 production. Homologs for this protein exist in humans and primates (129). Microinjection of cRNA encoding human PLCζ induced a prolonged series of calcium oscillations in aged human oocytes that closely mimicked the repetitive nature of the Ca(2+) stimulus provided by the sperm during human fertilization. At low concentrations, injection of PLCζ; induced parthenogenesis and development to the blastocyst stage (130). The use of sperm extracts and PLCζ to activate human oocytes for the purposes of generating both cloned and parthenogenetic stem cell lines certainly warrants further investigation.
Parthenogenetically activated oocytes themselves represent a possible source of pluripotential stem cell lines that are not embryonic. A nonhuman ESC line was established by Cibelli et al. (131,132) from parthenogenetic activation of monkey oocytes that showed all the characteristics of traditional human ESCs. Recently, Brevini et al. (133) have reported the production of human ESCs derived from parthenogenetically activated human oocytes. Oocytes were activated by 5µ Mionomycin (5 min) and 2 mM6-dimethylaminopurine (6-DMAP) (3 h). Two cell lines extensively proliferated as diploid, undifferentiated cells for more than 25 passages and maintained the expression of pluripotential markers. They showed differentiation as embryoid bodies in the three primary germ layers (endoderm, mesoderm, and ectoderm) in vitro. This data suggests that non embryonic ESCs can be established from unfertilized human oocytes and this should be further explored as a source of new pluripotent stem cells.
Human Embryo Culture
Culture environment during the preimplantation period of development profoundly influences the physiology and viability of mammalian embryos. Susceptibility to environmental stimuli decreases as development proceeds. The degree to which abnormal gene expression and altered imprinting patterns can be reduced or averted by using more physiological and environmental conditions is presently unknown. Improvements in the success of the assisted reproductive technologies for the treatment of infertility have coincided with the development of highly complex, biphasic culture media and culture conditions, which offer improved in vitro development, pregnancy rates, and live births (134). A multitude of commercially sourced media, manufactured under stringent manufacturing conditions, are available for this purpose. However, culture media specifically optimized for the development of SCNT is not yet available. Previous work indicates that NTSC embryos may require different culture requirements as a result of altered physiology and gene metabolism expression pathways (135). Thus, optimizing such culture conditions may be important for understanding the kinetics and mechanisms of nuclear reprogramming and to improving the over-all process.
Alternative Strategies for Therapeutic Cloning
Cell Fusion Based
The first demonstration that a pluripotent cell could reprogram a somatic cell following cell fusion to form a stable heterokaryon was in the mouse (136), and this phenomenon has been used to study nuclear reprogramming and gene expression (137). In an analogous human study, ESCs have recently been shown to efficiently reprogram human adult skin cells following cell to cell fusion (138). The current limitation remains the removal of the original ES cell nuclei to form a diploid isogenic ESC line, although recent work indicates that the use of centrifugation both before and after fusion can accomplish this task (139,140). Spontaneous heterokaryon formation is known to occur in vivo, as fused cell types have been observed following transplantation of bone marrow derived cells into adult mice brain and liver (141–143). Although this technology has the potential to replace the use of human oocytes in the reprogramming process, the low rates of spontaneous fusion, the inefficiency of producing large numbers of reprogrammed diploid cells, and a variety of safety concerns may ultimately limit its use (144).
Interspecies Therapeutic Cloning
The scarcity of human oocytes available for this type of research has led some researchers to generate human ESCs by reprogramming somatic cells with oocytes from another species (145). ESCs generated by this process appear to retain the characteristics of those derived from human embryos (145). The apparent universally conserved ability of oocyte components to reprogram gene expression in somatic cells is generating considerable interest leading to its exploration in several species (146–149). The utility of this approach requires further investigation. To overcome the concerns of mitochondrial heteroplasmy, it has been suggested that the ooplasm, mitochondria, as well as other ultrastructural components be replaced with human equivalents in a process similar to cytoplasmic transfer to allow a generation of blastocyst made up entirely of human material (8).
Extract-Mediated Transdifferentiation
The elucidation of the mechanisms associated with nuclear reprogramming could eventually lead to a direct reprogramming of human somatic cell nuclei without the use of eggs. Such an approach would ameliorate many of the difficulties associated with current nuclear transfer procedures and has-ten the generation of replacement cells for therapeutic purposes. The demonstration that adult stem cells have broader differentiation potential than anticipated and that they can contribute to tissues other than those in which they reside, has led to the development of a novel process that directly converts one somatic cell into another (a process known as transdifferentiation) (150,151). Functional reprogramming of a somatic fibroblast cell using a nuclear and cytoplasmic extract derived from another somatic cell type (T-cells) was demonstrated by nuclear uptake and assembly of transcription factors, induction of activity of a chromatin remodeling complex, changes in chromatin composition, and activation of lymphoid cell-specific genes. The reprogrammed cells expressed surface molecules specific to T-cells and exhibited apparently normal regulatory functions. In vitro cell reprogramming may also allow an examination of the mechanisms of nuclear reprogramming. Its applicability remains limited by the large amount of cell extract needed to reprogram a single cell; it is estimated that the extract from 300 cells is required to initiate transdifferentiation in a single cell. This approach may be useful in the identification and characterization of new molecules involved in the remodeling/reprogramming process.
Conclusions
Although therapeutic cloning for the purpose of creating patient-specific stem cells has considerable promise, its realization depends on the development of robust SCNT technologies utilizing human oocytes. Since the birth of the first mammalian clone almost 10 yr ago, the technique has been successfully accomplished in a wide range of mammalian species. Although the rapid rate of progress in this area is certainly impressive, both cloned embryos and offspring exhibit variations in regulation of gene expression as a consequence of the epigenetic interplay between the original somatic celland its new embryonic environment. As a result, only a small percent of cloned embryos result in viable offspring; however, surviving clones appear to have equivalent physiological and reproductive capacities when compared with their noncloned counterparts. As a result of these studies, a considerable body of work has been accumulated on the technical, biological, and molecular aspect of nuclear transfer in mammals. Key factors in the success of SCNT appear consistent across mammals and include cell type, degree of cell-cycle synchrony, effectiveness of parthenogenetic activation, and in vitro culture conditions.
Recent developments have focused on events surrounding reprogramming and the initiation of embryonic development in an effort to more closely replicate the well orchestrated events observed in more developmentally competent IVF and in vivo embryos. It appears that when fully optimized, in vitro development rates of cloned and parthenogenetic embryos may approach those of in vitro fertilized embryos, suggesting an important gauge of SCNT proficiency in any given species. Of increasing relevance to human therapeutic cloning, is that the efficiency of deriving ESC lines from cloned embryos is substantially more efficient than the production of viable offspring. Moreover, ES lines derived from cloned blastocysts appear to share identical characteristics to those derived from IVF embryos.
However, it is possible that a greater proportion of ESC lines might have a high rate of epigenetic errors because statistically many of the original embryos would not have resulted in viable animals. Nonetheless, recent reports (152) showing transcriptional profiles consistent with normal development of ES cell lines derived from cloned and fertilized mouse blastocysts, were functionally indistinguishable and had identical therapeutic potential. The derivation of ESC from cloned embryos appears to rigorously select for those immortal cells that have erased the “epigenetic memory” of the donor nucleus, in contrast to aberrant cloning phenotypes observed in the embryonic and fetal development of cloned animals (152).
Progress in SCNT in humans has been disappointing and is likely to be constrained by the ethical considerations involved in the procurement of high-quality oocytes. However, the enormous potential of patient-specific and disease-specific ESC lines has reinvigorated the research effort in human therapeutic cloning. With research burgeoning in this area, it seems it is only a matter of time before it will be successfully accomplished. The full realization of the therapeutic utility of NTSC is likely to be dependent on the ability to establish human ESC lines in defined conditions that allow the rapid multiplication of a purified population of an undifferentiated homogenous cell type, whereas avoiding contamination by animal products and pathogens.
Advances in human SCNT for the purpose of ESC line derivation are likely to result from a careful examination of ongoing research undertaken for mammalian reproductive cloning and other synergistic technologies associated with reprogramming. Hopefully, the use of this information will allow a higher level of efficiency for human therapeutic cloning and assist in developing guiding principles that will facilitate the accomplishment of autologous ESC lines for cell-based therapies (153–156). In the meantime, it is apparent that parthenogenetically activated oocytes are capable of producing nonembryonic pluripotential stem cells and this source of stem cells for therapeutic application should be explored in detail.
References
1. Cho MK, McGee G, Magnus D. Science 2006;311(5761): 614–615.
2. Lerou PH, Daley GQ. Blood Rev 2005;19(6):321–331.
3. Doss MX, Koehler CI, Gissel C, Hescheler J, Sachinidis A. J Cell Mol Med 2004;8(4):465–473.
4. Vats A, Tolley NS, Bishop AE, Polak JM. J R Soc Med 2005;98(8):346–350.
5. Trounson A, Pera M. Reprod Fertil Dev 1998;10(1):121–125.
6. Hall VJ, Stojkovic P, Stojkovic M. Stem Cells 2006;30:30.
7. Colman A, Kind A. Trends Biotechnol 2000;18(5):192–196.
8. Mollard R, Denham M, Trounson A. Differentiation 2002;70(1):1–9.
9. Stojkovic M, Stojkovic P, Leary C, et al. Reprod Biomed Online 2005;11(2):226–231.
10. Kues WA, Niemann H. Trends Biotechnol 2004;22(6):286–294.
11. Lewis IM, Munsie MJ, French AJ, Daniels R, Trounson AO. Reprod Med Rev 2001;9(1):3–33.
12.Somatic Cell Nuclear Transfer (Cloning) Efficiency. 2002. (Accessed at http://www.roslin.ac.uk/downloads/webtablesGR.pdf)
13. Vajta G, Gjerris M. Anim Reprod Sci 2006;92(3–4):211–230.
14. Faber DC, Ferre LB, Metzger J, Robl JM, Kasinathan P. Cloning Stem Cells 2004;6(2):198–207.
15. Lewis IM, French AJ, Tecirlioglu RT, et al. Aust J ExpAgric 2004; 44:1105–1111.
16. Lewis IM, McClintock AE, French AJ, Zuelke KA, Harford BA, Trounson AO. Aust Vet J 2000;78(10):694–697.
17. Paterson L, DeSousa P, Ritchie W, King T, Wilmut I. Anim Reprod Sci 2003;79(3–4):137–143.
18. Wells DN. Reprod Suppl 2003;61:131–150.
19. Wells DN. Rev Sci Tech 2005;24(1):251–264.
20. Wells DN, Oback B, Laible G. Trends Biotechnol 2003;21(10): 428–432.
21. Oback B, Wells D. Cloning Stem Cells 2002;4(2):169–174.
22. Oback B, Wells D. Cloning Stem Cells 2002;4(2):147–168.
23. Oback B, Wells DN. Cloning Stem Cells 2003;5(4):243–256.
24. Kishigami S, Wakayama S, van Thuan N, Wakayama T. Hum Cell 2006;19(1):2–10.
25. Wang L, Duan E, Sung LY, Jeong BS, Yang X, Tian XC. Biol Reprod 2005;73(1):149–155.
26. Rideout WM, 3rd, Hochedlinger K, Kyba M, Daley GQ, Jaenisch R. Cell 2002;109(1):17–27.
27. Wakayama S, Jakt ML, Suzuki M, et al. Stem Cells 2006;24:2030–2033.
28. Wakayama T, Tabar V, Rodriguez I, Perry AC, Studer L, Mombaerts P. Science 2001;292(5517):740–743.
29. Wakayama S, Kishigami S, Van Thuan N, et al. Proc Natl Acad Sci USA 2005;102(1):29–33.
30. Wakayama S, Mizutani E, Kishigami S, et al. J Reprod Dev 2005; 51(6):765–772.
31. Wakayama S, Ohta H, Kishigami S, et al. Biol Reprod 2005; 72(4):932–936.
32. Wakayama T. Nat Biotechnol 2004;22(4):399–400.
33. Wilmut I, Schnieke AE, McWhir J, Kind AJ, Campbell KH. Nature 1997;385(6619):810–813.
34. Kato Y, Tani T, Tsunoda Y. J Reprod Fertil 2000;120(2):231–237.
35. Humpherys D, Eggan K, Akutsu H, et al. Proc Natl Acad Sci USA 2002;99(20):12,889–12,894.
36. Wakayama T, Rodriguez I, Perry AC, Yanagimachi R, Mombaerts P. Proc Natl Acad Sci USA1999;96(26):14,984–14,989.
37. Wells DN, Misica PM, Day AM, Peterson AJ, Tervit HR. Reprod Fertil Dev 1998;10(7–8):615–626.
38. Miyashita N, Shiga K, Yonai M, et al. Biol Reprod 2002; 66(6):1649–1655.
39. Gong G, Dai Y, Zhu H, et al. Sci China C Life Sci 2004;47(5):470–476.
40. Wakayama T, Rodriguez I, Perry AC, Yanagimachi R, Mombaerts P. Proc Natl Acad Sci USA 1999;96(26):14,984–14,989.
41. Wells DN, Forsyth JT, McMillan V, Oback B. Cloning Stem Cells 2004;6(2):101–110.
42. Hochedlinger K, Jaenisch R. N Engl J Med 2003;349(3):275–286.
43. Reik W, Dean W, Walter J. Science 2001;293(5532):1089–1093.
44. Dean W, Santos F, Stojkovic M, et al. Proc Natl Acad Sci USA 2001;98(24):13,734–13,738.
45. Bourc’his D, Le Bourhis D, Patin D, et al. Curr Biol 2001; 11(19):1542–1546.
46. Kang YK, Koo DB, Park JS, et al. Nat Genet 2001;28(2):173–177.
47. Santos F, Zakhartchenko V, Stojkovic M, et al. Curr Biol 2003;13(13):1116–1121.
48. Xue F, Tian XC, Du F, et al. Nat Genet 2002;31(2):216–220.
49. Nolen LD, Gao S, Han Z, et al. Dev Biol 2005;279(2):525–540.
50. Daniels R, Hall V, Trounson AO. Biol Reprod 2000;63(4): 1034–1040.
51. Humpherys D, Eggan K, Akutsu H, et al. Science 2001;293(5527): 95–97.
52. Rideout WM, 3rd, Eggan K, Jaenisch R. Science 2001;293(5532): 1093–1098.
53. Wrenzycki C, Wells D, Herrmann D, et al. Biol Reprod 2001;65(1): 309–317.
54. Yanagimachi R. Mol Cell Endocrinol 2002;187(1–2):241–248.
55. Daniels R, Hall VJ, French AJ, Korfiatis NA, Trounson AO. Mol Reprod Dev 2001;60(3):281–288.
56. Boiani M, Eckardt S, Scholer HR, McLaughlin KJ. Genes Dev 2002;16(10):1209–1219.
57. Chavatte-Palmer P, Remy D, Cordonnier N, et al. Cloning Stem Cells 2004;6(2):94–100.
58. Heyman Y, Richard C, Rodriguez-Martinez H, et al. Cloning Stem Cells 2004;6(2):111–120.
59. Shiga K, Umeki H, Shimura H, Fujita T, Watanabe S, Nagai T. Theriogenology 2005;64(2):334–343.
60. Tecirlioglu RT, Cooney MA, Korfiatis NA, et al. Theriogenology 2005;12:12.
61. Korfiatis NA, Hall VJ, Ruddock NT, Lewis IM, French AJ. Blastocyst development rates from in vitro produced, nucleartransfer andparthenote bovine embryos: Effect of oocyte quality and/or maturation. In; 2002: Theriogenology; 2002; pp. 532.
62. Lonergan P, Rizos D, Gutierrez-Adan A, Fair T, Boland MP. Reprod Domest Anim 003;38(4):259–267.
63. Blondin P, Bousquet D, Twagiramungu H, Barnes F, Sirard MA. Biol Reprod 2002;66(1):38–43.
64. Campbell KH, Alberio R. Reprod Suppl 2003; 61:477–494.
65. Campbell KH, Loi P, Otaegui PJ, Wilmut I. Rev Reprod 1996;1(1): 40–46.
66. Urakawa M, Ideta A, Sawada T, Aoyagi Y. Theriogenology 2004;62(3–4):714–728.
67. Sullivan EJ, Kasinathan S, Kasinathan P, Robl JM, Collas P. Biol Reprod 2004;70(1):146–153.
68. An International Consortium on Stem Cells, Ethics and Law: Consensus Statement 2006. (Accessed at www.hopkinsmedicine.org/bioethics.)
69. Committee on Guidelines for Human Embryoninc Stem Cell Research BoLS, Division on Earth and Life studies, Board on Health Sciences Policy, Institute of Medicine. Guidelines for Human Embryonic Stem Cell Research. Washington DC: National Academies Press; 2005.
70. Hansen JE. J Med Ethics 2002;28(2):86–88.
71. de Wert G, Mummery C. Hum Reprod 2003;18(4):672–682.
72. Dhai A, Moodley J, McQuoid-Mason DJ, Rodeck C. S Afr Med J 2004;94(11):906–909.
73. Larijani B, Zahedi F. Transplant Proc 2004;36(10):3188–3189.
74. Munsie MJ, Michalska AE, O’Brien CM, Trounson AO, Pera MF, Mountford PS. Curr Biol 2000;10(16):989–992.
75. Kawase E, Yamazaki Y, Yagi T, Yanagimachi R, Pedersen RA. Genesis 2000;28(3–4):156–163.
76. Barberi T, Klivenyi P, Calingasan NY, et al. Nat Biotechnol 2003;21(10):1200–1207.
77a. Lavoir MC, Weier J, Conaghan J, Pedersen RA. Reprod Biomed Online 2005;11(6):740–744.
77b. Cibelli JB, Lanza RP, West MD, Ezzell C. Sci Am 2002; 286:44–51.
77c. Lu CF, Lin G, Xie CQ, et al. Chinese Science Bulletin 2003;48: 1840–1843.
78. Simerly C, Navara C, Hyun SH, et al. Dev Biol 2004;276(2):237–252.
79. Simerly CR, Navara CS. Cloning Stem Cells 2003;5(4):319–331.
80. Meng L, Ely JJ, Stouffer RL, Wolf DP. Biol Reprod 1997; 57(2):454–459.
81. Macklon NS, Stouffer RL, Giudice LC, Fauser BC. Endocr Rev 2006;27(2):170–207.
82. Healy DL, Trounson AO, Andersen AN. Lancet 1994;343(8912): 1539–1544.
83. Blyth E. Hum Reprod 2002;17(12):3254–3259.
84. Heng BC. Reprod Biomed Online 2005;11(6):676–678.
85. Trounson A, Anderiesz C, Jones GM, Kausche A, Lolatgis N,
Wood C. Hum Reprod 1998;13(Suppl 3):52–62; discussion 71–75.
86. Cha KY, Chian RC. Hum Reprod Update 1998;4(2):103–120.
87. Friden B, Hreinsson J, Hovatta O. Hum Reprod 2005;20(9): 2556–2558.
88. Biron-Shental T, Fisch B, Van Den Hurk R, Felz C, Feldberg D, Abir R. Fertil Steril 2004;81(3):716–719.
89. Kuwayama M, Vajta G, Kato O, Leibo SP. Reprod Biomed Online 2005;11(3):300–308.
90. Heng BC. Reprod Biomed Online 2006;12(3):280–281.
91. Hubner K, Fuhrmann G, Christenson LK, et al. Science 2003;300(5623):1251–1256.
92. Lacham-Kaplan O, Chy H, Trounson A. Stem Cells 2006; 24(2):266–273.
93. Kehler J, Hubner K, Garrett S, Scholer HR. Semin Reprod Med 2005;23(3):222–233.
94. Gurdon JB, Byrne JA, Simonsson S. Novartis Found Symp 2005;265:129–136; discussion 36–41, 204–211.
95. Tamada H, Van Thuan N, Reed P, et al. Mol Cell Biol 2006;26(4):1259–1271.
96. Kikyo N, Wade PA, Guschin D, Ge H, Wolffe AP. Science 2000;289(5488):2360–2362.
97. Gurdon JB. J Biosci 2005;30(1):11–14.
98. Alberio R, Johnson AD, Stick R, Campbell KH. Exp Cell Res 2005;307(1):131–141.
99. Gao S, Chung YG, Williams JW, Riley J, Moley K, Latham KE. Biol Reprod 2003;69(1):48–56.
100. Sutovsky P, Moreno RD, Ramalho-Santos J, Dominko T, Simerly C, Schatten G. Biol Reprod 2000;63(2):582–590.
101. Kaneda H, Hayashi J, Takahama S, Taya C, Lindahl KF, Yonekawa H. Proc Natl Acad Sci USA 1995;92(10):4542–4546.
102. Ashley MV, Laipis PJ, Hauswirth WW. Nucleic Acids Res 1989;17(18):7325–7331.
103. Howell N, Halvorson S, Kubacka I, McCullough DA, Bindoff LA, Turnbull DM. Hum Genet 1992;90(1–2):117–120.
104. Laipis PJ, Van de Walle MJ, Hauswirth WW. Proc Natl Acad Sci USA 1988;85(21):8107–8110.
105. St John JC, Lloyd RE, Bowles EJ, Thomas EC, El Shourbagy S. Reproduction 2004;127(6):631–641.
106. Steinborn R, Schinogl P, Zakhartchenko V, et al. Nat Genet 2000;25(3):255–257.
107. Hiendleder S, Schmutz SM, Erhardt G, Green RD, Plante Y. Mol Reprod Dev 1999;54(1):24–31.
108. Takeda K, Takahashi S, Onishi A, Goto Y, Miyazawa A, Imai H. J Reprod Fertil 1999;116(2):253–259.
109. Evans MJ, Gurer C, Loike JD, Wilmut I, Schnieke AE, Schon EA. Nat Genet 1999;23(1):90–93.
110. Tecirlioglu RT, Cooney MA, Lewis IM, et al. Reprod Fertil Dev 2005;17(5):573–585.
111. Brenner CA, Kubisch HM, Pierce KE. Reprod Fertil Dev 2004;16(7):743–751.
112. Santos TA, El Shourbagy S, St John JC. Fertil Steril 2006;85(3):584–591.
113. Alberio R, Zakhartchenko V, Motlik J, Wolf E. Int J Dev Biol 2001;45(7):797–809.
114. Swann K. Development 1990;110(4):1295–1302.
115. Sato K, Fukami Y, Stith BJ. Semin Cell Dev Biol 2006;17(2):285–292.
116. Machaty Z, Prather RS. Reprod Fertil Dev 1998;10(7–8):599–613.
117. Ozil JP. Development 1990;109(1):117–127.
118. Ozil JP, Huneau D. Development 2001;128(6):917–928.
119. Winston N, Johnson M, Pickering S, Braude P. Fertil Steril 1991;56(5):904–912.
120. Balakier H, Casper RF. Hum Reprod 1993;8(5):740–743.
121. Rinaudo P, Pepperell JR, Buradgunta S, Massobrio M, Keefe DL. Fertil Steril 1997;68(6):1086–1092.
122. Cibelli JB, Kiessling AA, Cuniff K, Richards C, Lanza RP, West MD. J Regenerative Med 2001;2:25–31.
123. Nakagawa K, Yamano S, Nakasaka H, Hinokio K, Yoshizawa M, Aono T. Zygote 2001;9(1):83–88.
124. Lin H, Lei J, Wininger D, et al. Stem Cells 2003;21(2):152–161.
125. Homa ST, Swann K. Hum Reprod 1994;9(12):2356–2361.
126. Wu H, He CL, Fissore RA. Mol Reprod Dev 1998;49(1):37–47.
127. Saunders CM, Larman MG, Parrington J, et al. Development 2002;129(15):3533–3544.
128. Swann K, Saunders CM, Rogers NT, Lai FA. Semin Cell Dev Biol 2006;17(2):264–273.
129. Cox LJ, Larman MG, Saunders CM, Hashimoto K, Swann K, Lai FA. Reproduction 2002;124(5):611–623.
130. Rogers NT, Hobson E, Pickering S, Lai FA, Braude P, Swann K. Reproduction 2004;128(6):697–702.
131. Cibelli JB, Grant KA, Chapman KB, et al. Science 2002;295(5556):819.
132. Vrana KE, Hipp JD, Goss AM, et al. Proc Natl Acad Sci USA 2003;100(Suppl 1):11,911–11,916.
133. Brevini V, Tosetti M, Crestan M, Paffoni A, Ragni G, Gandolfi F. Derivation and characterization of parthenogenetic human embryonic stem cells. In: 22nd Annual Meeting of the European Society of Human Reproduction and Embryology; 2006 June; Prague: Human Reproduction 21(Suppl 1) Abstract O-238; 2006; pp. i93.
134. Gardner DK, Lane M. Hum Reprod 1998;13(Suppl 3):148–159; discussion 60.
135. Chung YG, Mann MR, Bartolomei MS, Latham KE. Biol Reprod 2002;66(4):1178–1184.
136. Tada M, Tada T, Lefebvre L, Barton SC, Surani MA. EMBO J 1997;16(21):6510–6520.
137. Ambrosi DJ, Rasmussen TP. J Cell Mol Med 2005;9(2):320–330.
138. Cowan CA, Atienza J, Melton DA, Eggan K. Science 2005; 309(5739):1369–1373.
139. Strelchenko N, Kukharenko V, Shkumatov A, Verlinsky O, Kuliev A, Verlinsky Y. Reprod Biomed Online 2006;12(1):107–111.
140. Pralong D, Mrozik K, Occhiodoro F, et al. Cloning Stem Cells 2005;7(4):265–271.
141. Weimann JM, Johansson CB, Trejo A, Blau HM. Nat Cell Biol 2003;5(11):959–966.
142. Wang X, Willenbring H, Akkari Y, et al. Nature 2003;422(6934): 897–901.
143. Vassilopoulos G, Wang PR, Russell DW. Nature 2003;422(6934): 901–904.
144. Vassilopoulos G, Russell DW. Curr Opin Genet Dev 2003;13(5): 480–485.
145. Chen Y, He ZX, Liu A, et al. Cell Res 2003;13(4):251–263.
146. Murakami M, Otoi T, Wongsrikeao P, Agung B, Sambuu R, Suzuki T. Cloning Stem Cells 2005;7(2):77–81.
147. Zhong ZS, Zhang G, Meng XQ, et al. Exp Cell Res 2005;306(1):35–46.
148. Dindot SV, Farin PW, Farin CE, et al. Biol Reprod 2004;71(2): 470–478.
149. Kitiyanant Y, Saikhun J, Chaisalee B, White KL, Pavasuthipaisit K. Cloning Stem Cells 2001;3(3):97–104.
150. Collas P, Hakelien AM. Trends Biotechnol 2003;21(8):354–361.
151. Hakelien AM, Collas P. Cloning Stem Cells 2002;4(4): 379–387.
152. Brambrink T, Hochedlinger K, Bell G, Jaenisch R. Proc Natl Acad Sci USA 2006;103(4):933–938.
153. Grimaud C, Negre N, Cavalli G. Chomosome Res 2006;14(4):363–375.
154. Morgan HD, Santos F, Green K, Dean W, Reik W. Hum Mol Genet 2005;(Spec No 1914):R47–R58.
155. Trounson A. Endocr Rev 2006;27(2):208–219.
156. Trounson AO. Reprod Fertil Dev 2001;13(7–8):523–532.
For more information, please contact:
Roman Jimenez 858-453-2305
Downloading articles requires Adobe Reader. Don't have it? Click here.
